
Professonal Interests
The aim of our laboratory is to develop novel vaccine candidates against infectious diseases such as malaria, which is outstanding among domestic pharmaceutical institutes. The researches are focusing on the biology of malaria parasites, the basic researches about infectious diseases and molecular immunology, and those clinical outputs such as vaccine developments and innovative drug developments. Our long-term goal is to develop more highly effective next generation vaccines against malaria, raising an army of "exploitation of a novel pharmaceutical research" at the laboratory of biological pharmacy.
【研究テーマ】
 I. Malaria Vaccine Developments
I. Malaria Vaccine Developments
II. Development of anti-malaria transmission blocking vaccine
III. Nasal drop vaccines –Effective novel vaccine platform
IV. Novel drug developments of the anti-platelet reagent AAPP
V. Development of an immune-epidemiological biomarker to evaluate the efficacy of malaria vector control interventions
VI. Development of non-human virus-based vectors for liver-directed gene therapy
VII. Bispecific single-chain antibody fragment as a new concept anti-malaria drug
VIII. Elucidation of the malaria parasite-mosquito salivary gland interaction using a robust salivary gland specific transgene expression system
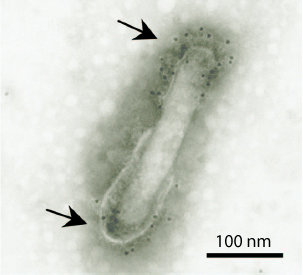 I. Malaria Vaccine Developments : This study investigates the development of novel vaccine candidates using a bacurovirus-display vector. The vector exhibits malaria antigens such as merozoite surface protein 1 and circumsporozoite protein on the viral envelope. It is of interest that immunization with the vaccine provided 100% protection against murine Plasmodium spp. From a biological safety perspective, this attractive malaria vaccine has several advantages, including (i) low cytotoxicity, (ii) an adjuvant-free platform, (iii) an inability to replicate in mammalian cells, and (iv) an absence of preexisting antibodies. In collaboration with a pharmaceutical company, our research is centered on understanding the molecular mechanisms of the baculovirus-based vaccine in the pre-clinical trials. We never stop hoping to start human clinical trials for this malaria vaccine.
I. Malaria Vaccine Developments : This study investigates the development of novel vaccine candidates using a bacurovirus-display vector. The vector exhibits malaria antigens such as merozoite surface protein 1 and circumsporozoite protein on the viral envelope. It is of interest that immunization with the vaccine provided 100% protection against murine Plasmodium spp. From a biological safety perspective, this attractive malaria vaccine has several advantages, including (i) low cytotoxicity, (ii) an adjuvant-free platform, (iii) an inability to replicate in mammalian cells, and (iv) an absence of preexisting antibodies. In collaboration with a pharmaceutical company, our research is centered on understanding the molecular mechanisms of the baculovirus-based vaccine in the pre-clinical trials. We never stop hoping to start human clinical trials for this malaria vaccine.
Yoshida S, Kawasaki M, Hariguchi N, Hirota K, Matsumoto M.
A baculovirus dual expression system-based malaria vaccine induces strong protection against Plasmodium berghei sporozoite challenge in mice.
Infect Immun 77:1782-9, 2009.
Yoshida S, Kondoh D, Arai E, Matsuoka H, Seki C, Tanaka T, Okada M, Ishii A.:
Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protects mice against malaria sporozoite infection.
Virology 316: 161-70, 2003.
PTC/JP2007/51295「Novel vaccine vector」(2007) Yoshida et al.
II. Development of anti-malaria transmission blocking vaccine (TBV) - Study of transmission blocking vaccines against malaria parasite in the mosquito stage: This study investigates an anti-malaria transmission blocking vaccine (TBV) that prevents fertilization and/or ookinate/oocyst development within the mosquito is an attractive strategy to limit the transmission of malaria. To examine transmission blockade in vivo, baculovirus vaccine (BBV) immunized mice were infected malaria, A. stephensi mosquitoes were fed directly on the immunized and challenged hosts. Active immunization of mice followed by challenge demonstrates a strong transmission-blocking response, with reduction in oocyst intensity. This TBV will target in the mosquito stage of the parasite and prevent the spread of malaria through the community; such a vaccine would have the potential to reduce the burden of disease and death from malaria, including in parts of the world’s most malarious continent, Africa. The aforementioned (1), it has been shown that BBV-based vaccines are indeed effective against pre-erythrocytic and erythrocytic stage. Here, TBV demonstrates feasibility of immunization against transmission stages. Thus, BBV-based vaccines offer a promising new alternative to current human vaccine delivary platforms and possess a great potential for the development of multi-stage vaccine. 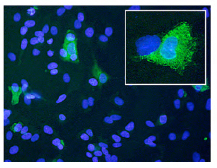
Mlambo G, Kumar N, Yoshida S.
Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen.
Vaccine 28:7025-9, 2011.
Blagborough AM, Yoshida S, Sattabongkot J, Tsuboi T, Sinden RE.
Intranasal and intramuscular immunization with Baculovirus Dual Expression System-based Pvs25 vaccine substantially blocks Plasmodium vivax transmission.
Vaccine 28:6014-20, 2010.
III. Nasal drop vaccines –Effective novel vaccine platform : Nasal drop vaccines have several attractive features such as safety, cost-effectiveness, and ease of administration. Due to a needle-free platform, nasal vaccination could be the optimal type of administration in malarial areas. This novel platform could induce not only local mucosal immune responses but also systemic and protective immune responses against the parasites. Intranasal immunization with BBV, our vaccine system, resulted in the sterile immunity against murine malaria parasites, indicating the superiority to other immunization regimens. This study focuses on the molecular mechanisms underlying the protective efficacy by the nasal immunization, which plays a pivotal role for the clinical setting.
Yoshida S, Nagumo H, Yokomine T, Araki H, Suzuki A, Matsuoka H.
Plasmodium berghei circumvents immune responses induced by merozoite surface protein 1- and apical membrane antigen 1-based vaccines.
PLoS One 5:e13727, 2010.
Yoshida S, Araki H, Yokomine T.
Baculovirus-based nasal drop vaccine confers complete protection against malaria by natural boosting of vaccine-induced antibodies in mice.
Infect Immun 78:595-602, 2009.
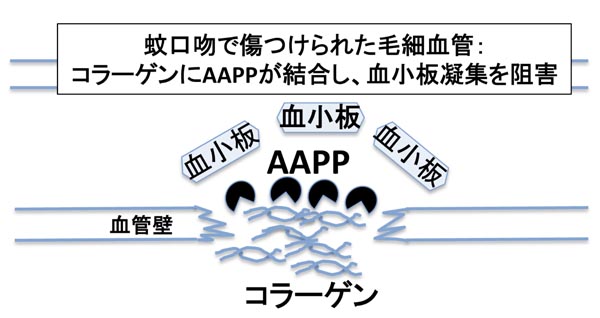 IV. Novel drug developments of the anti-platelet reagent AAPP: Why can mosquitoes feed on humans? During blood vessels injured, platelets can be accumulated into the sites with collagens, and then fixed. But, the Anopheles mosquitoes can stick the needle several times into human skins and successfully take the blood. We discovered that a protein found in the female salivary glands has a capability of inhibiting platelet aggregation via a collagen activation pathway. We renamed the protein as “Anopheline antiplatelet protein (AAPP)” and designed its clinical application as an anti-platelet agent in collaboration with a pharmaceutical company. In addition, the study may also provide important insights for elucidating the effects of mosquito blood feeding against host hemostasis.
IV. Novel drug developments of the anti-platelet reagent AAPP: Why can mosquitoes feed on humans? During blood vessels injured, platelets can be accumulated into the sites with collagens, and then fixed. But, the Anopheles mosquitoes can stick the needle several times into human skins and successfully take the blood. We discovered that a protein found in the female salivary glands has a capability of inhibiting platelet aggregation via a collagen activation pathway. We renamed the protein as “Anopheline antiplatelet protein (AAPP)” and designed its clinical application as an anti-platelet agent in collaboration with a pharmaceutical company. In addition, the study may also provide important insights for elucidating the effects of mosquito blood feeding against host hemostasis.
Hayashi H, Kyushiki H, Nagano K, Sudo T, Iyori M, Matsuoka H, Yoshida S.
Identification of the active region responsible for the anti-thrombotic activity of anopheline anti-platelet protein from a malaria vector mosquito.
Platelets in press, 2012.
Hayashi H, Kyushiki H, Nagano K, Sudo T, Matsuoka H, Yoshida S.
Anopheline anti-platelet protein from a malaria vector mosquito has anti-thrombotic effects in vivo without compromising hemostasis.
Thromb Res 129(2):169-75, 2012.
Yoshida S, Sudo T, Niimi M, Tao L, Sun B, Kambayashi J, Watanabe H, Enjou L, Matsuoka H.
Inhibition of collagen-induced platelet aggregation by anopheline anti-platelet protein, a saliva protein from a malaria vector mosquito.
Blood 111:2007-14, 2008.
PCT/JP2006/322417号「Anti-Platelet Aggregation Product」 (2006) Yoshida et al.
V. Development of an immune-epidemiological biomarker to evaluate the efficacy of malaria vector control interventions : This study investigates a potential immunological maker, based on human antibody responses to Anopheles saliva protein AAPP, as a new indicator to evaluate the efficacy of vector-control strategies. For the fight against malaria, many preventing interventions have been integrated against both parasite (chemoprophylaxis) and vector (insecticide-based control of either larvae and adults). Among such strategies, insecticide-treated nets (ITNs) are currently the most efficient at reducing vector exposure, Plasmodium transmission, and malaria morbidity. Evaluating ITNs efficacy is currently based on entomological methods (mosquito abundance, blood feeding rates, mortality, etc) and in human, parasitological tests. However, these methods have certain limitations when many other innervations are set up at the same time. Therefore, the World Health Organization (WHO) has emphasized the need for the indicators to evaluate and compare the efficacy of each vector-control strategies. In a malaria hyperendemic area, where inhabitants were naturally bitten by anopheline mosquitoes over 100 bites per night, we have found the positive correlation between prevalence of antibodies to AAPP and malaria infection. At present, in collaboration with the Indonesia government, we are aiming to develop the AAPP biomarker for the evaluation of new vector-control strategies (ITNs use, house-hold spraying, curtains, etc).
VI. Development of non-human virus-based vectors for liver-directed gene therapy : This study develops baculovirus-based vectors targeting liver for gene therapy. Malaria sporozoites, when injected into human body by blood meals of mosquito, reach quickly to liver through blood flow and invade specifically into hepatocytes. Using those specific targeting mechanisms of sporozoites to liver, we are developing gene delivery vector specific for liver. We would offer these vectors as novel therapeutics method for hemophilia, liver cancer, metabolic abnormalities et.al in future.
VII. Bispecific single-chain antibody fragment as a new concept anti-malaria drug : This study investigates a potential of bispecific single-chain antibody fragment as an anti-malaria drug. OKT3 is a monoclonal antibody against ε subunit of human CD3 complex. 5.2 is a monoclonal antibody that recognizes an epitope within the 19 kDa C-terminal region of Plasmodium falciparum MSP-1 (PfMSP-1),. Both genes encoding OKT and 5.2 mAb were cloned and assembled as a single gene encoding 5.2-OKT3 bispecific single-chain antibody fragment (biscFV) in which scFV of OKT3 is fused to scFV of 5.2. 5.2-OKT3 biscFV recognizes both of CD3ε and MSP-1 and induces aggregation of merozoites around T cells. Importantly, in P. faliciparum in vitro culture in the presence of human lymphocytes, when aggregation, not only IL-2, IFN-γ and TNF-α cytokines are produced from T cells, but also significant increase of merozoite phagocytosis by monocytes is observed. At present, we are further investigating in vivo therapeutic effect of modified biscFVs on blood-stage transgenic P. berghei expressing PfMSP-1 in a murine model.
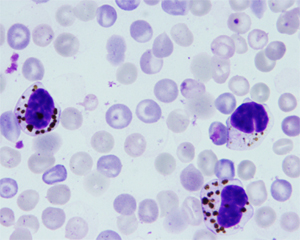 Figure: Enhancement of phagocytic activities of macrophages by 5.2-OKT3 biscFv
Figure: Enhancement of phagocytic activities of macrophages by 5.2-OKT3 biscFv
Yoshida S, Kobayshi T, Matsuoka H, Seki C, Gosnell WL, Chang SP, Ishii A.
T cell activation and cytokine production via a bispecific single-chain antibody fragment targeted to blood-stage malaria parasites.
Blood 101: 2300-2306, 2003.
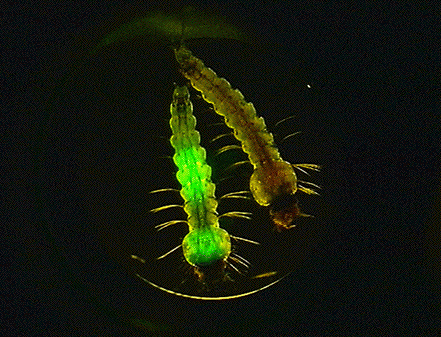
VIII. Elucidation of the malaria parasite-mosquito salivary gland interaction using a robust salivary gland specific transgene expression system : This study investigates the malaria parasite-mosquito salivary gland interaction using a robust salivary gland specific transgene expression in Anopheles stephensi. Malaria sporozoites invade the mosquito salivary glands and wait in the salivary duct until the next blood feeding. The mechanisms of the process and molecules involved in the salivary gland invasion remain largely unknown. Recent advances in the genetic engineering of anopheline mosquitoes have raised hopes for their use as new strategies for malaria control and provided a powerful tool to investigate the mosquito–parasite interactions. For the past decades, it has been postulated that establishment of salivary gland-specific gene expression would make it possible to elucidate salivary gland–parasite interactions and contribute to the biological control of this important infectious disease; for example, through the generation of a transgenic mosquito refractory to the parasites. In 2006, we established for the first time the robust salivary gland-specific expression system in transgenic Anopheles stephensi. Using this system, we have established five lines of transgenic mosquitoes, which express an anti-sporozoite effector, a Leishmania vaccine candidate antigen, etc.
 Figure: Transgenic Anopheles stephensi
Figure: Transgenic Anopheles stephensi
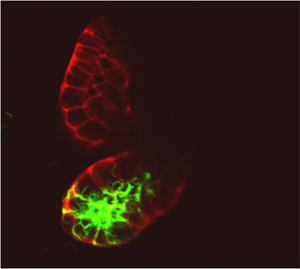 Figure: GFP-epressing parasites in transgenic mosquitoes
Figure: GFP-epressing parasites in transgenic mosquitoes
Sumitani M, Kasashima K, Yamamoto DS, Yagi K, Yuda M, Matsuoka H, Yoshida S.
Reduction of malaria transmission by transgenic mosquitoes expressing an antisporozoite antibody in their salivary glands.
Insect Mol Biol In press, 2012.
Yamamoto DS, Sumitani M, Nagumo H, Yoshida S, Matsuoka H.
Induction of antisporozoite antibodies by biting of transgenic Anopheles stephensi delivering malarial antigen via blood feeding.
Insect Mol Biol 21:223, 2012.
Yamamoto DS, Nagumo H, Yoshida S.
Induction of antisporozoite antibodies by biting of transgenic Anopheles stephensi delivering malarial antigen via blood feeding.
Insect Mol Biol 19:391, 2010.
Yoshida S, Shimada Y, Kondoh D, Kouzuma Y, Ghosh AK, Jacobs-Lorena M, Sinden RE.
Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development.
PLoS Pathogen 3:e192, 2007.
Yoshida S & Watanabe H.
Robust salivary gland-specific transgene expression in Anopheles stephensi mosquito.
Insect Mol Biol 15:403-10, 2006.
Copyright© Laboratory of Vaccinology and Applied Immunology, Kanazawa University, 2011-2020.
金沢大学医薬保健研究域薬学系 ワクチン・免疫科学研究室
     |